Food Science Corner: What are Fats and Oils?
Last Updated on February 17, 2023
Fat, it comes in many forms: margarine, lard, shortening and oil. It provides richness, flavour and structure to all your recipes. However, if you choose the incorrect fat for your recipe then this can mean disaster!
To me it seems that everywhere I turn the media depicts fats as bad for your health but this really isn’t the case. We shouldn’t be scared of fats because we need them to support healthy cells and bodies. Fats provide us with energy and help us to adsorb fat-soluble vitamins like A,D,E and K. [5] But remember like everything the key is moderation with your intake.
Regardless, I am not here to talk to you about the health benefits of fats. The field of nutrition is a tricky one and that side of the industry is not in my wheelhouse. Instead, I want to focus on something that is– the chemistry!
So let’s start with the big question:
What are fats and how are they different than oils?
Fats and oils are part of a group of compounds known as lipids. [3] In biology and biochemistry, a lipid is a macro biomolecule that is soluble in nonpolar solvents [4]. Lipids are a huge group of molecular compounds that include triacylglycerols, diacylglycerols, monoacylglycerols, free fatty acids, sterols, and phospholipids. [3]
Although the terms are used interchangeably fats are lipids that are solid at room temperature while oils are lipids that are liquid at room temperature.
When talking to food scientists and they call something a “fat” they typically are referring to the class of molecules known as triacylglycerols (TAGs). TAGs consist of a glycerol skeleton where each hydroxyl group is esterified to a fatty acid (FA).
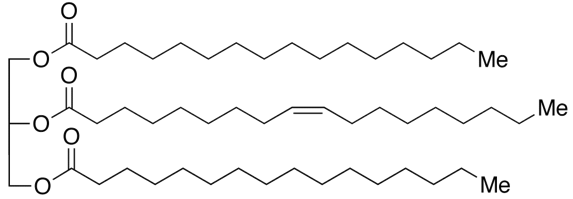
1,3-dipalmitoyl-2-oleoyl-glycerol (POP) – a major triglyceride found in cocoa butter
So how does one TAG differ from the next?
Well TAGs differ from one another based on the chemical structure of the fatty acid chains. A fatty acid chain is a carboxylic acid with a long aliphatic chain which is either saturated or unsaturated (but I will get into what that is later). In the example photo above you can see there are three fatty acid chains in a TAG.
The molecular structure/name of the TAG species can be attributed to:
- The total carbon number on the FA where the total carbon number (CN) is the sum of the alkyl chain lengths of each of the three FAs
- The degree of unsaturation in each FA
- The position and the configuration of the double bonds in the FAs
So how does changing these properties above actually affect your cooking? Well let’s start with the overall structure.
Effect of Composition on Fat Properties
Fatty Acid Chain Length
How long the fatty acid chains are in the the TAG will play a role in how hard or soft your fat is. Softer/ more liquid fats have a higher ratio of short-chain fatty acids with lower melting points compared to long-chain fatty acids. [2] Overall, the melting points of saturated fatty acids rise as the number of carbons increase.
This can be seen in the figure below by Ernesto M. Hernandez, Afaf Kamal-Eldin, and Ernesto M. Hernandez which show the relation between melting point and the number of carbon atoms in saturated fatty acids. [2]
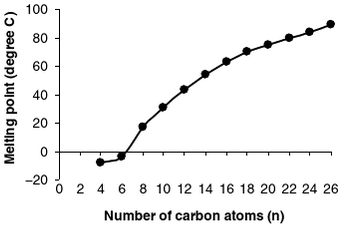
However, keep in mind that when we talk about a food source not all the molecules will be the same. So your block of lard does not contain all the same molecule but instead a mixture. So even though I am referring to TAGs (which make up the bulk of the mixture) there are other compounds which exist in the mixture.
Degree of Unsaturation
Another property of fatty acids the determines its physical properties is the saturation of the molecules. In other words, triglycerides can have areas of unsaturation within their fatty acid chains. [2] Some carbon atoms are linked by single bonds (-C-C-) and others are linked by double bonds (-C=C-). Saturation refers to the maximum possible number of hydrogen atoms are bonded to each carbon in the molecule. If your fatty acid chain contains no double bonds that it is saturated. If it does contain double bonds than your fatty acid is unsaturated.
Now when you introduce an unsaturated bond to the mixture these fat can start to “zig-zag” where the carbons can sit either in a “cis” or “trans” position. This positioning will affect how the molecules pack together and plays a role in the softness or hardness of a compound. However, I will talk more about this in another article when I talk about fat crystallization.
Chains that are trans saturated fatty acid chains are able to pack more efficiently than short-kinked (cis) unsaturated fatty acid chains they have higher melting points and contribute to solid-like functionality. [1]
Position of the unsaturated bond
Finally, where the double bond is located in the fatty acid chain will affect it’s melting point. Both cis– and trans-C18 monounsaturated are higher melting when the double bond is at even positions than at odd positions. [4]
The thing I want you to remember most of all from this portion above is that:
The higher the melting point a fat has the harder the fat is
Hydrogenation
Because the degree of saturation affects the melting points of fats some manufactures will add hydrogen to an oil to saturate it. This process is known as hydrogenation.
When you partially hydrogenate something it allows for an oil to become a semisolid even at room temperature. This is where health concerns come in. Studies suggest that partially hydrogenated fats like trans fats change plasma lipid levels negative ways and cause inflammation in arteries. [6] Again I don’t want to get into the health issues but there is a growing body of evidence that you should avoid hydrogenated trans fats and still to unsaturated fats such as those found in fish such as Omega-3 fatty acids.
Role of lipids and fats in foods
As mentioned before, the type of fat you choose for cooking is dependent on what you are cooking. Fats are used extensively in cooking because there extensive properties allow them to be. So let’s break it down and talk about that.
Flavour
An obvious but important role. Fats have the ability to absorb and preserve flavours. Fat simply taste good and are able to provide recipes with a more pleasant mouth feel and taste.
Solvent
Firstly, fats can be used as a solvent for non-water soluble compounds like vitamins, antioxidants, preservatives. For example, vitamin D is an oil soluble vitamin that is fat soluble and not water soluble. That means that you need to have fat in the mixture in order for the vitamin to be dissolved in it.
Tenderization
Fats are able to make baked goods tender by impeding the formation of gluten. When you have enough fat in a recipe they can shorten the gluten strands causing your baked goods to be tender and flakey- think pie crusts and biscuits. If you didn’t add the fat the gluten strands would be able to grow long providing strength and a chewy texture to bread. However, a little bit of fat allows for lubrication making it easier for the gluten network to expand.
Creaming
Creaming is a technique known in baking where you soften butter and blend it with other ingredients. The main goal during creaming is to incorporate air into your baking batter. During the baking process (putting it in the oven) the fat melts but the air which is trapped in the batter is able to cause the baked food to lighten or rise.
Heat Transfer
When sautéing vegetables or garlic the first thing that you add to the plan is oil. The reason you do this is because fats are one of the most efficient modes of heat transfer during cooking. Not only does it prevent your food from sticking to the plan it also can prevent food to overheat to the interior portions.

Author: Veronica Hislop is a Master’s thesis student in the Molecular Science program at Ryerson University. She is also a career partner with FoodGrads and has work experience in the food processing industry working both in R&D and QA.
Currently, she is performing research on water-in-oil emulsions stabilized by fat crystals. When she is not following her scientific endeavors you can find her enjoying Japanese anime, manga and video games.
Subscribe to our newsletter for details on mentorship sessions, workshops, webinars, as well as career and job fairs across Canada and the US!
References
[1] Linear and nonlinear rheological behavior of fatcrystal networks
[2] Hernandez, E. M. (Ed.). (2013). Processing and nutrition of fats and oils. ProQuest Ebook Central https://ebookcentral-proquest-com.ezproxy.lib.ryerson.ca
[3] Emulsion p
[4] IUPAC, Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”) (1997). Online corrected version: (2006–) “lipids“. doi:10.1351/goldbook.L03571
[5] How should we judge edible oils and fats? An umbrella review of the health effects of nutrient and bioactive components found in edible oils and fats
[6] The negative effects of hydrogenated trans fats and what to do about them

leave your comment