Food Science Corner: What is Texture?
Last Updated on February 17, 2023
Taste is king. However, in the world of food texture is next in line for the throne. Enjoying food isn’t just limited to the taste but how it feels in the mouth. We use the term mouthfeel to describe the textural properties of food as perceived in the mouth. However, this is a subjective measurement and people have differences in how they perceive texture. For instance, someone who has dental issues might perceive food as being crunchier than those without them.
Although we are aware of the texture of foods; how do we do this with a more measurable and scientific method? Well, we subject food to measurable forces and see how the food responds to it. Therefore, scientists study the consistency and flow of food under specified conditions under the study rheology.
What is Rheology?
Rheology is the science of flow and deformation of solid-like and liquid-like materials. [1] When subjected to external forces, solids (or truly elastic materials) will deform, whereas liquids (or truly viscous materials) will flow. It is not just limited to the study of foods but is instead a whole field of research. Rheology is used in many fields such as the manufacture of paints and plastics. Being able to predict the rheology of a material is important in judging the quality of it.
For example, using a tool known as a rheometer you can determine if the paint you are going to apply to a wall has a high level of flow or not. You wouldn’t want the paint to be too thick or it would be cakey on your walls. That wouldn’t be a nice look. Therefore, rheometers are used extensively for quality control.
Foods generally exist on a viscoelastic spectrum having some liquid and solid properties. Water can be considered an ideal liquid with 100% liquid properties because it flows when you pour it out of a glass. In contrast, a rubber band is an example of an ideal solid material where if you apply a deformation it will go back to it’s original shape. To my knowledge, there is no food that is considered an ideal solid material.
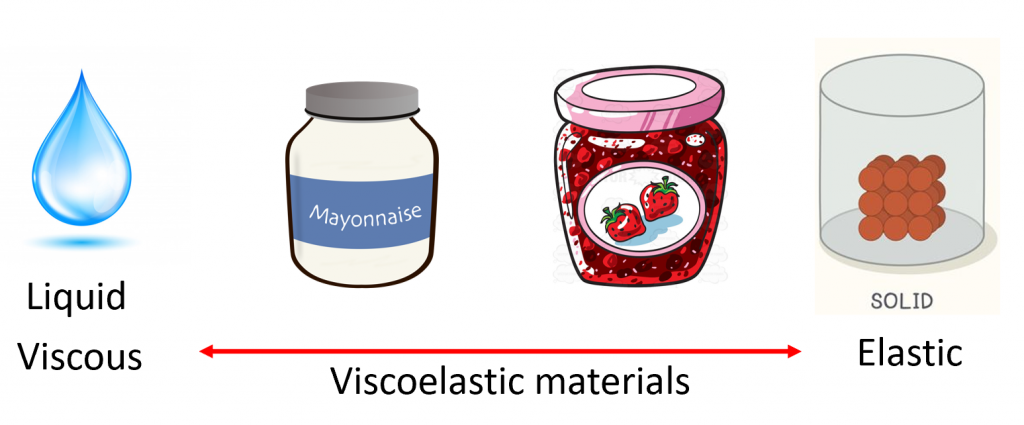
However, most foods are not ideal liquids or solids and instead exist on the spectrum where they have liquid and solid-like characteristics. They are neither completely elastic nor entirely viscous. Ketchup for example is viscoelastic material because it will not flow out of the jar unless you add an external force such as tapping on the bottle.
To better understand these properties and why they happen scientists turn to rheology and different kinds of rheological tests. One of the most well-known ones is viscosity.
What is viscosity?
By definition viscosity is a measure of a fluid’s resistance to deformation at a given rate. [2] In other words viscosity gives information on how “thick” a fluid is and how easily it flows. A “thin” or low viscosity product is milk while a “thick” or high viscosity liquid is honey. Viscosity is measured in the units of Pascals per second (Pa/s) where the larger the value the more “thick” your substance is.
Approximate Viscosities of Common Materials (At Room Temperature-70°F) *
| Material | Viscosity in Centipoise |
|---|---|
| Water | 1 cps |
| Milk | 3 cps |
| Castrol Oil | 1,000 cps |
| Karo Syrup | 5,000 cps |
| Honey | 10,000 cps |
| Chocolate | 25,000 cps |
| Ketchup | 50,000 cps |
| Mustard | 70,000 cps |
| Sour Cream | 100,000 cps |
| Peanut Butter | 250,000 cps |
Why is it important to know what a product’s viscosity is?
Consumer’s and manufacturers expect products to stay consistent. A chocolate manufacture would want to know the viscosity of molten chocolate when it flows through the pipes of a manufacturing factory. If the chocolate was too thick because it got cold it could become stuck within the pipes at a manufacturing plants. In contrast, a consumer would not want their mayonnaise to be too thin or it would fall right off the bread!
Generally, higher viscosity fluids have more complex and larger molecules. Starches, proteins, hydrocolloids and gums are all ingredients that cause fluids to become thicker. [3]
For example, jams are thick because they contain an ingredient known as pectin which contains large protein chains with numerous chemical groups (hydroxyl groups, anionic groups etc.). Chemical groups which are hydrophilic (water-loving) can bind to water molecules causing the proteins to swell and immobilize water. A network is than created which holds water in it’s place causing an increase in viscosity which in turn causes is less movement from the water in the compound.
Another thing we must keep in mind is viscosity is not a definitive property and that it can change under different condition such temperature, shear rate and stress. So let’s take a minute to define what shear rate and shear stress are.
What is shear stress?
Shear stress is an amount of force per unit area (=Force/Area) where the units of force are in N, newton and shear area are in m2. We can think of stress as a normalized force, i.e., force acting on a unit area. Think of shear stress as the intensity of force. We can apply stress in different directions.
It is important to consider how much force we apply to a system because it will affect it’s properties. Going back to the ketchup example, if you only apply a small amount of shear stress the ketchup will not flow but if you increase it significantly the ketchup will begin to flow out of the bottle quite easily.
So overall, stress is just applying applying force to something.

Do you ever find you can’t get the ketchup out of the bottle but then all of a sudden with one final tap it goes everywhere?
However, this is just the case for liquids. If you were apply stress to a solid it will experience deformation. If you lightly tap jello it will return to its original shape. It will temporarily deform but than go back to it’s original shape. However, if you add enough of a stress than you can permanently deform the system where it does not go back to it’s original shape.

The more solid your food product the more it is able to resist deformation!
Just remember:
For solids there is deformation but for liquids the stress is used to determine when it flows
Equation for Shear Rate
Complimentary to shear stress is shear rate. However, to understand this concept we first need to think of a model.
Imagine that you have two plates in parallel (one on top of the other) with a gap between the two. The bottom plate stays stationary while the top one can in move in parallel to the lower plate (slide). The shear rate describes the rate at which the two plates slide past each other.
Mathematically shear rate is defined as:
Shear rate = v / h
Where v is the velocity (in m/s) and h (is the height between the two plates).
The faster the one plate moves past the bottom the faster your shear rate is. In practical terms, we can also think about the shear rate in the context of mixing something with a spoon, the faster you stir it the higher the shear rate.
Bringing it back to viscosity
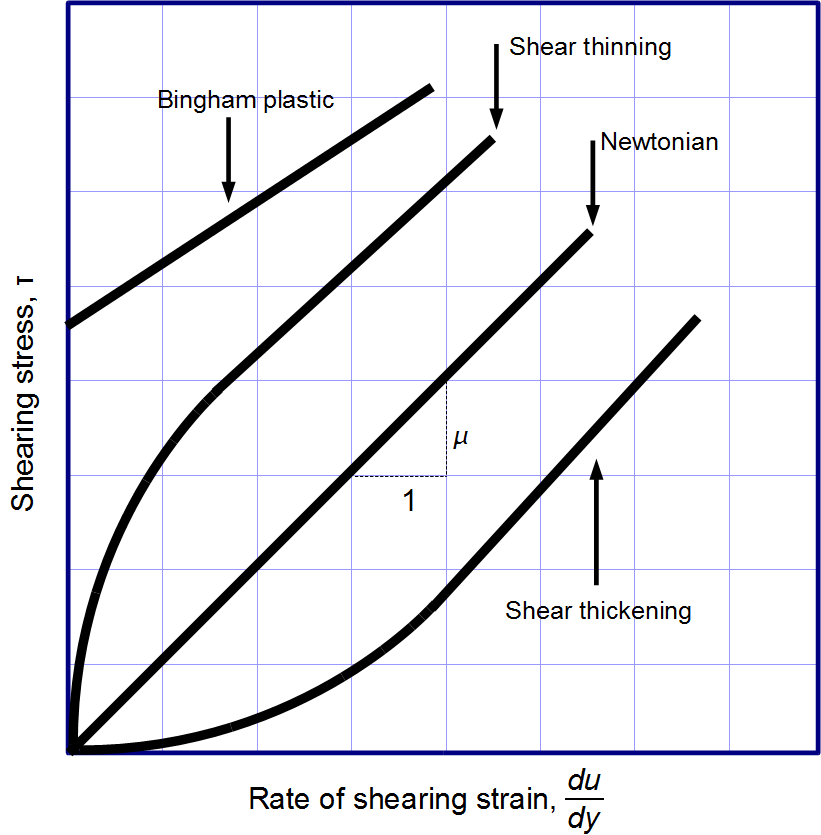
Now that we have defined these terms we can go back to viscosity. Mathematically, viscosity = shear stress / shear rate. Therefore, a compound can experience different properties depending on how you change these variables. However, not every compound acts the same because of their molecular composition.
For example, water will have the same viscosity whether you mix it at 600m/s or 1m/s but not all compounds are like that. Skim milk for example is a mixture of fat globules and milk proteins which are dispersed in water. This emulsion experiences “shear-thinning” where as you increase the shear rate the apparent viscosity decreases. [2] This is common to a lot of foods but in rare cases you fill find a material that gets thicker as you stir it faster.
One of the few foods that experience shear thicken is corn starch. the faster you stir it the thicker the material becomes. At low shear forces, a suspension of corn starch and water behaves as a liquid, where it will flow, take the shape of its container, and resist compression. However, if you were to mix the material really fast it would get thicker. This is due to the starch molecules which are present in the mixture.
As you can see from the graph above there are different types of materials and they will act differently depending on how fast you mix them! [3]
What is texture then?
Now that we better understand all these definitions we can finally get to the bottom of this article and understand what is texture?
According to the International Standards Organization (ISO) texture is defined as the “All the rheological and structure (geometrical and surface) attributes of a food product perceptible by means of mechanical, tactile, and where appropriate, visual and auditory receptors” (ISO 5492, 2008).
Foods can be crunchy, smooth, rough and so much more! Texture is a blanket term that can be used to describe anything from how a food feels in your mouth to how it looks.
Texture can be thought of as an index of quality. You can judge whether vegetables are still good based on their firmness. If fruits or vegetable lose water during storage they wilt or lose their turgor pressure. This is what causes your crisp apple to become mushy and taste bad. [4]
A dishes textural experience changes depending on what stage we are in the easting process. The eating experience can be broken down into 6 steps according to Dar and Light (2014) [5]
- Appearance: Staring with visual contact with the food. That may sound odd but think about when you judge food on Instagram. You already begin to make judgements of the texture just by looking at the photo. Does ice cream look gritty or smooth because of it’s shine?
- Touch: Touch occurs when you first interact with the food. When putting the spoon through the ice cream. How easily does it flow through? Is it sticky? Rough?
- First Bite: This occurs when you put the food in your mouth. How is that first bite? (A very important step!) The first bite in your mouth can direct the entire eating experience. Does it crunch when you bite down? How loud is it when you crunch it?
- Mastication: Any chewing that happens after the first bite is mastication. Key texture attributes include resistance to chewing, the way the food breaks down in the mouth, the extent to which it coasts the mouth, how it sticks to your teeth or how long it takes to break down in your mouth.
- Swallowing: How food feels going down your throat. Does it hurt such as when you don’t chew enough? Foods can be rough going down your throat or even thick such as when swallowing pills.
- Residual: The final step of the eating experience! Did the food leave any residue after you have consumed the food such as a film being left in your mouth from an oily food.
Overall, rheology is a fascinating field full of interesting concepts and ways to explore food. It is a cornerstone of my thesis. Stay tuned to learn more science behind my thesis with upcoming posts!

Author: Veronica Hislop is a Master’s thesis student in the Molecular Science program at Ryerson University. She is also a career partner with FoodGrads and has work experience in the food processing industry working both in R&D and QA.
Currently, she is performing research on water-in-oil emulsions stabilized by fat crystals. When she is not following her scientific endeavors you can find her enjoying Japanese anime, manga and video games.
Subscribe to our newsletter for details on mentorship sessions, workshops, webinars, as well as career and job fairs across Canada and the US!
References
[1] The basics of rheology by Anton Paar https://wiki.anton-paar.com/en/basics-of-rheology/
[2] Solanki, G., & Rizvi, S. S. H. (2001). Physico-Chemical Properties of Skim Milk Retentates from Microfiltration. Journal of Dairy Science, 84(11), 2381–2391. https://doi.org/10.3168/jds.s0022-0302(01)74687-5
[3] Crawford, N. C., Popp, L. B., Johns, K. E., Caire, L. M., Peterson, B. N., & Liberatore, M. W. (2013). Shear thickening of corn starch suspensions: Does concentration matter? Journal of Colloid and Interface Science, 396, 83–89. https://doi.org/10.1016/j.jcis.2013.01.024
[4] Potter, N. N., & Hotchkiss, J. H. (1998). Food science (5th ed.). Springer.
[5] Dar, Y., & Light, J. M. (2014). Food texture design and optimization. Wiley.

leave your comment