Food Science Corner: What are emulsions?
Last Updated on February 21, 2023
Salad dressing, margarine, butter and milk are all examples of emulsions that you would find in the kitchen.
But what is an emulsion?
An emulsion is a temporarily stable mixture of two immiscible (unmixable) liquids such as oil and water. Liquid droplets (the disperse phase) are dispersed into another liquid medium (the continuous phase) such as oil in water. 1
A simple example of a temporary emulsion is a mixture of oil and water. If you poured oil and water into a bowl and whisked it like crazy you would end making a ton of tiny droplets. The liquid droplets become suspended within each other and by definition, this is an emulsion.
Though what is happening here? How can these two things come together? We generally recognize that water and oil do not mix. There are two main reasons for this.
- Water and oil do not have the same densities. Water is denser than oil so when the two are combined water sinks while the oil floats. This is due to the difference in mass and size of the molecules.
- Water is a polar substance while oil is a non-polar substance. Because of this water molecules stick together. Polar molecules only dissolve in polar substances. Therefore, oil and water do not mix.2
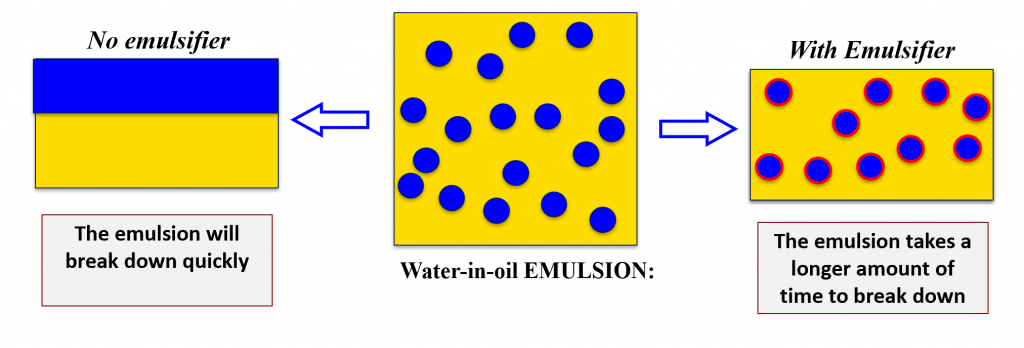
When mixing the two ingredients together you are adding energy into the system allowing for a temporary combination of the two. However, given enough time the emulsion will break down (separate) because this state is not favourable for the mixture.
Oil and water want to touch each other as little as possible so they come up with an orientation that limits their interactions. What eventually happens is that the ingredients go back to being two distinct layers.
This orientation is the most energetically favourable for oil and water. As well, it also has the lowest surface tension-the tendency of liquid surfaces to shrink into the minimum surface area possible.
To overcome this surface tension and allow for ingredients to be mixed for longer periods of time a third component is needed – emulsifiers!
What are emulsifiers?
Emulsifiers (also referred to as a surface-active agents or surfactants) are compounds that stabilizes emulsions. They increase the kinetic stability of a system by reducing the surface tension between the two unmixable ingredients.1
Emulsifiers are able to do this because of their molecular structures. Emulsifiers consist of a polar and non-polar region. The polar portion is referred to as the hydrophilic region (water-loving) because it has a strong affinity for water while the non-polar region is known as the hydrophobic region because it is water rejecting.
Emulsifiers act as physical barriers preventing the components in emulsions from coming together by creating a physical film between the components.4 Going back to the water and oil example, an emulsifier would create a physical barrier between the the two liquids mitigating the amount they touch each other. Therefore, the water and oil are able to co-exist in the same mixture. All because the emulsifier was able to increase the kinetic stability of the system.
Common emulsifiers in food
One size does not fit all when it comes to choosing an emulsifier for an emulsion. You need to consider what ingredients you are mixing and the proportions as well. Is the emulsion you are creating an oil-in-water emulsion or a water-in-oil emulsion?
Common emulsifiers which are used in the food industry are3:
- Egg yolks- Egg yolks contain lecithin
- Lecithin- Lecithin can also be extracted from the oil of seeds such soybeans
- Mono- and diglycerides – emulsifiers derived from fatty acids
- Pickering particles – solid particles
- Polysorbates
With all these examples how are you supposed to choose the best one for you? Well as a home cook you generally don’t have these emulsifiers to choose from. They are mainly used as functional ingredients in the food industry. For example, Polyglycerol polyricinoleate (PGPR) is used in the chocolate industry to help with the thickness of chocolate.5
The ones that you are more more likely to use in the kitchen include egg yolks, butter (whey protein and casein act as emulsifiers), cheese and mustard (contains the pectin rhamnogalacturonan).6
HLB Values
In the food industry, researchers will choose emulsifiers based on their HLB value. The semiempirical scale for selecting surfactants was developed by Griffin. Hydrophilic-lipophile balance (HLB) describes the degree at which a surfactant is soluble in either water or oil.
How much a surfactant dissolves is based on the size of the hydrophilic and hydrophobic portions or the surfactant.
High HLB values are associated with high water solubility. Therefore, they are useful when preparing and stabilizing oil-in-water emulsions. An example of an emulsifier with a high HLB value is polysorbate 80 which is used to control fat agglomeration in ice cream and whipped toppings. Low HLB values emulsifiers are associated with high oil solubility which is useful when formulating of water-in-oil (W/O) emulsions such as margarine.

References
- Tadros, T. F. Emulsion Formation, Stability, and Rheology. In Emulsion Formation and Stability; 2013. https://doi.org/10.1002/9783527647941.ch1.
- Sell, C. Fundamentals of Fragrance Chemistry, 1st ed.; Wiley-VCH: Weinheim, 2019.
- Wilbey, R. A. Emulsifiers in Food Technology. Int. J. Dairy Technol. 2006. https://doi.org/10.1111/j.1471-0307.2006.00224.x.
- Ghosh, S.; Rousseau, D. Fat Crystals and Water-in-Oil Emulsion Stability. Curr. Opin. Colloid Interface Sci. 2011, 16 (5), 421–431. https://doi.org/10.1016/j.cocis.2011.06.006.
- Afoakwa, E. O.; Paterson, A.; Fowler, M. Effects of Particle Size Distribution and Composition on Rheological Properties of Dark Chocolate. Eur. Food Res. Technol. 2008. https://doi.org/10.1007/s00217-007-0652-6.
- Cui, W.; Eskin, M. N. A.; Biliaderis, C. G.; Marat, K. NMR Characterization of a 4-O-Methyl-β-D-Glucuronic Acid-Containing Rhamnogalacturonan from Yellow Mustard (Sinapis Alba L.) Mucilage. Carbohydr. Res. 1996. https://doi.org/10.1016/0008-6215(96)00158-9.

Author: Veronica Hislop – Veronica is a Master’s thesis student in the Molecular Science program at Ryerson University. She is also a career
partner with FoodGrads and has work experience in the food processing industry working both in R&D and QA.
Currently, she is performing research on water-in-oil emulsions stabilized by fat crystals. When she is not following her scientific endeavors you can find her enjoying Japanese anime, manga and video games.
Subscribe to our newsletter for details on mentorship sessions, workshops, webinars, as well as career and job fairs across Canada and the US!


[…] researcher that studies the texture of emulsions like butter or margarine for example may use tools such as texture analyzers, microscopes, X-ray […]