Food Science Corner: The Chemistry of Water
Last Updated on February 17, 2023
Water, the thing we need to survive. It makes up all life here on earth and an necessity in understanding if you want to make tasty food! When it comes to cooking some recipes call for a lot of water (ex. soups) while others only require a small amount (ex. crackers).
Almost all food processing techniques involve the use of water in some respect to another. Whether it is in the form of freezing, drying, emulsification, thickening of starch or making pectin gels. Water plays a role in all of these processes. [1]
However, in order to understand these processes we need to understand the fundamental science behind the process- water! Let’s go!
What is water?
A simple question on the surface but the answer can be as simple or complex as you want it to be. Let’s start with the simple first.
Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms. It has a chemical formula of H2O and it’s while its dihydrogen oxide. The molecular name, however, makes it seem scarier than it actually is. After all, water is essential to life on Earth and is an important ingredient in food!
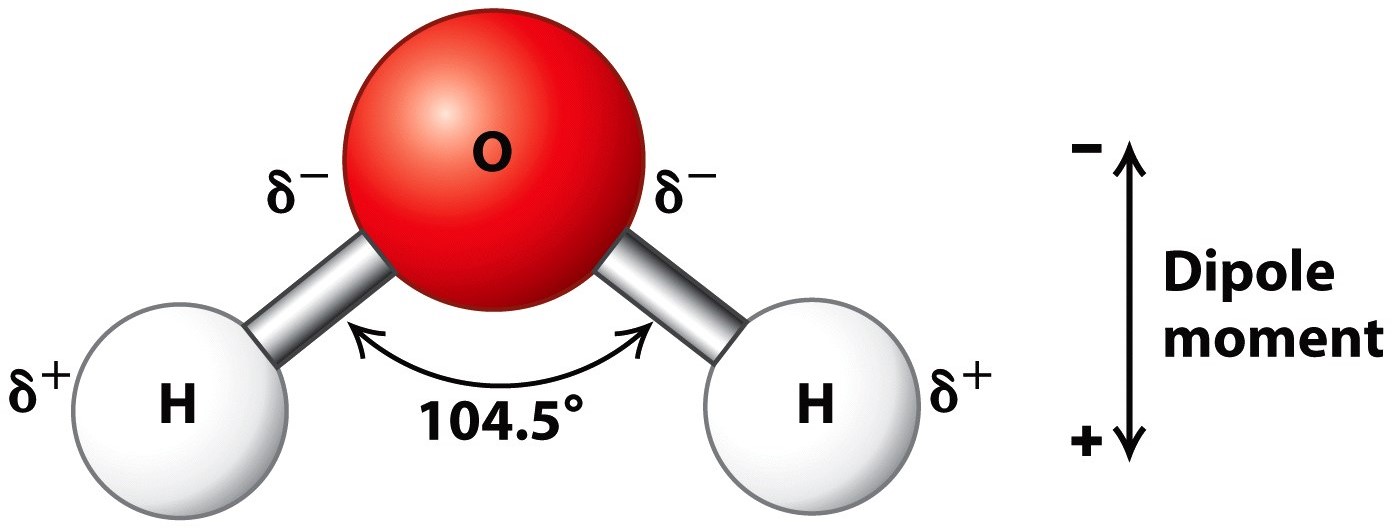
One of the major sources of water’s impressive properties comes from it’s ability to hydrogen bond.
Hydrogen bonding is a special type of dipole-dipole attraction between molecules, not a covalent bond to a hydrogen atom. [2]
Oxygen is highly electronegative and thus pulls the electrons from the hydrogen towards it. This allows for a partial positive charge on each of the hydrogen atoms and a partial negative charge on the lone pairs of electrons on the oxygen atom.
Due to this partial electronegativity, water can hydrogen bond between molecules. The lone pair of electrons on the oxygen atoms form a bond with a hydrogen atom on a neighbouring water molecule.
Due to water’s tetrahedral structure, it can hydrogen bond with multiple molecules. Therefore, one water molecule can hydrogen bond with four other molecules (in the solid-state). In the liquid state because there is a lot more movement and thermal energy this number becomes 3 to 3.5 at room temperature and increasing as the temperature goes up. [3]
Though hydrogen bonding isn’t just limited to just other water molecules. Water can also form bonds with other polar molecules, such as organic acids, bases, proteins and carbohydrates. [3]
Water boiling and freezing points
When working with water in your cooking there are two factors which you have to consider- it’s boiling point and freezing points.
Water has a boiling point at 100° C (212oF) and a freezing point at 0° C (32oF).
Assuming you are at sea level. However, these temperatures will change depending on where you live in the world. These changes are due to atmospheric pressures that are experienced at different elevations

However, what is truly unique about water is that when water freezes its density actually decreases and its volume increases by about 9%. [1]
This is why although counterintuitive that when you place an ice cube in water it floats rather then sinks despite putting a solid in a liquid.

Think about when you make ice cubes. Have you ever noticed that the water in the container appears to get larger? This is because of the number of hydrogen bonds that a water molecule makes when a solid as opposed to a liquid.
Hydrogen bonding is also the culprit when it comes to water’s high boiling point. Water requires a high input of energy in order to raise it’s temperature.
Water hardness
When talking about water it can be seen that all water is the same. After all it is just H2O – right? Well not necessarily, unless you only use distilled water from the grocery store.
Water which comes from your taps can include dissolved solutes like chlorine or calcium. Depending on where you live water can be obtained from surface or groundwater sources. For example, in the Greater Toronto Area the water from our taps comes from Lake Ontario. [4]
Different sources will have different levels of calcium or magnesium which is in turn is used to describe the hardness of water.
The hardness of water is defined in terms of its calcium and magnesium ion content. The hardness of water is measured in parts per million or in “grains” with one grain equivalent to 0.0064 g of calcium carbonate. [5]
Actually when I was an undergraduate I performed a lab experiment where I did a titration experiment where I used a chelating agent EDTA to see how hard the water was!
In food science, you have to be aware of the hardness of your water because it will play a role in how your cooking turns out- especially baking!
For example, the hardness of water will affect the fermentation of bread Yeast growth is affected by the amount of minerals and nutrients in water. A change in the fermentation will affect the dough characteristics, making them stronger or weaker.
Hard water: High mineral content. When used in baking, dough exhibits high strength and fast fermentation.
Soft water: Low mineral content. When used in baking, dough exhibits low strength and slow fermentation. May need yeast to improve fermentation. [6]
If your water is too hard you can actually soften it by boiling it a by scaling soluble bicarbonate precipitate and causing it to scale. However, permanently hard water cannot be softened by boiling it as it contains either calcium or magnesium sulfates. [5]
So next time you are baking you might want to consider how hard is your water!
Author: Veronica Hislop

Veronica is an MSc Candidate in the Molecular Science program at Ryerson University. She is also the host of the FoodGrads podcast and career partner with FoodGrads. Veronica has work experience in the food processing industry working both in R&D and QA.
Currently, she is performing research on water-in-oil emulsions stabilized by fat crystals. When she is not following her scientific endeavors you can find her enjoying Japanese anime, manga and video games.
Subscribe to our newsletter for details on mentorship sessions, workshops, webinars, as well as career and job fairs across Canada and the US!
References
| (1) | Vaclavik, V. A.; Christian, E. W. Essentials of Food Science; Springer: New York, NY, 2008. |
| (2) | https://www.chem.purdue.edu/gchelp/liquids/hbond.html |
| (3) | McClements, D. J. Food Emulsions: Principles, Practices, and Techniques, Third Edition, 3rd ed.; CRC Press: London, England, 2015. https://doi.org/10.1201/b18868. |
| (4) | https://www.toronto.ca/services-payments/water-environment/tap-water-in-toronto/fast-facts-about-the-citys-water-treatment-plants/ |
| (5) | Gandhi, K.; Sharma, R.; Gautam, P. B.; Mann, B. Chemical Quality Assurance of Milk and Milk Products; Springer Singapore: Singapore, 2020. |
| (6) | (5) Water https://bakerpedia.com/ingredients/water/ (accessed Dec 22, 2020). |


leave your comment