Exploring the Fascinating Science of Eggs
Last Updated on July 28, 2024
Chicken eggs have been a cherished culinary tradition dating back to 1400 B.C.E. These versatile delectables serve various functions in recipes. Eggs can strengthen baked goods, add nutritional content to protein shakes, and even act as an emulsifier in ice creams. The science of eggs can be looked at through the lenses of composition, nutritional content, shelf life, food-borne illness risk and hard-boiling eggs. This article is going to be examining each of these scientific topics!
Egg Composition
Eggs have a protective outer shell made from calcium carbonate, which includes a semipermeable membrane and a bloom, the outermost coating.
The membrane consists of a strong inner and outer layer made from a class of proteins known as keratin, effectively safeguarding the egg from bacterial threats. At the bottom of the egg lies an air cell, creating a crater-like shape when boiled. Moving on to the egg whites, they are composed of a class of proteins called albumen. Eggs also contain chalazae, the strings of egg white which attach to the egg yolk’s casing and align with the eggshell’s membrane.
The vitelline membrane, a clear-coloured case, encloses the yolk, whose colour can vary based on the hen’s diet and variety. Carotenoids are responsible for giving egg yolks their colour. Carotenoids can be divided into two main groups- carotenoids and xanthophylls. Carotenes provide a red hue to eggs, while xanthophylls impart a yellow hue. Organic eggs, influenced by increased lycopene found in tomato powder from the chicken’s diet may exhibit a reddish hue in their yolks.
Lutein is just one of the pigments responsible for the yellow hue in eggs
Nutritional Content of Egg Yolks vs Whites
The composition of eggs may explain why some people prefer yolks vs whites when choosing certain diets. Yolks have a higher fat content than the same amount of egg whites, making their higher fat content perfect for use in rich custards. In addition, egg yolks are rich in minerals such as phosphorus and selenium. Alongside fat-soluble vitamins like vitamins E and K. On the other hand, egg whites are high in protein meaning they do well to supplement protein intake for athletes and bodybuilders.
Science Behind the Egg Shelf Life
The float test is a commonly used trick to determine the freshness of eggs. Fresh eggs will sink to the bottom, while older eggs will float to the top. These differences in buoyancy happen because as eggs age, the yolks and whites begin to decompose, producing gases that increase the size of the air pocket inside the shell. As time passes, these gases escape, reducing the egg’s mass and causing its density to be lower than water’s. Consequently, older eggs with a smaller density float on top, whereas fresh eggs that have not undergone this gas production remain at the bottom.
However, it is important to note that the float test does not necessarily indicate if the eggs have spoiled. To detect any signs of spoilage, notice any foul odours or visually inspect the eggs for cracks in the shell, a slimy texture, or powdery residues on the surface to help determine spoilage.
Eggs Food-borne Illness Risk
It is commonly recommended for individuals use pasteurized eggs when preparing dishes with raw eggs due to the risk of Salmonella bacterial contamination. The most common types of bacteria responsible for this are Salmonella typhimurium and Salmonella enteritidis, which can lead to symptoms like diarrhea, vomiting, and abdominal cramps. Notably, the Food and Drug Association has deemed pasteurized eggs safe for raw consumption. Pasteurization is a process that involves heating the eggshells at a specific time and temperature to kill the bacteria present. Despite the heating process, the eggs remain uncooked under these circumstances.
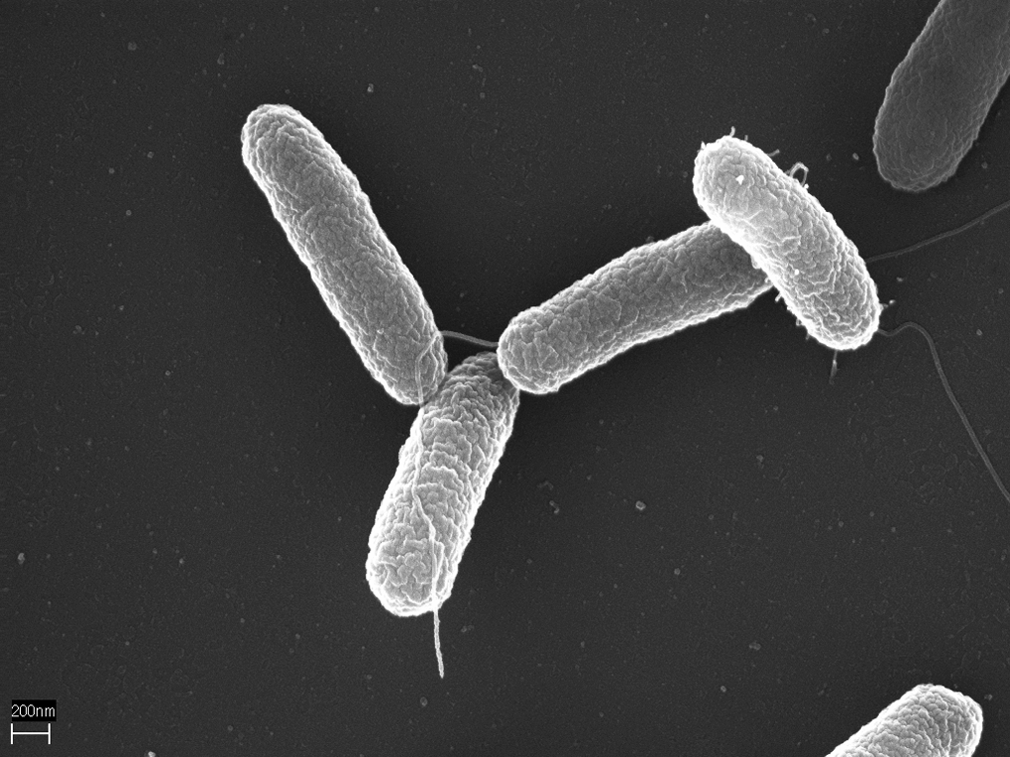
You don’t want to finding these in your eggs! [Source: Volker Brinkmann]
Science of Hard-Boiled Eggs
When eggs (shell included) are boiled long enough both the whites and yolks will solidify and thus are called hard-boiled eggs. This solidification results from the biochemical process of denaturation while the egg is heated. The heat causes folded albumen’s secondary, tertiary, and quaternary protein structures to unfold, breaking covalent bonds. This results in water becoming trapped, and the eggs solidify.
It is common to observe the formation of a green layer between the egg yolk and white when hard-boiling eggs. The green layer is caused by a reaction between iron ions within the yolk and hydrogen sulphide gas emitted from the whites, forming iron sulphide. Additionally, peeling hard-boiled eggs can become more or less challenging depending on the storage life of the eggs. Over time, eggs lose carbon dioxide through their pores, causing the pH to rise from 7.6 to 9.2. This change makes the albumen stick more to the interior of the shell, making it easier to peel.
Conclusion
Therefore, understanding the science of eggs introduces their anatomy, nutritional information, shelf life, potential biological hazards, and cooking properties. Thanks to their diverse food chemical functions, eggs remain a versatile ingredient in cooking.

About the Author: Hi! I am Bertina Do, a first-year food science student attending the University of Guelph. I have a fascination with food chemistry and molecular gastronomy.
Photo Reference
Photo: Salmonella typhimurium– Volker Brinkmann, Max Planck Institute for Infection Biology, Berlin, Germany, CC BY 2.5 https://creativecommons.org/licenses/by/2.5, via Wikimedia Commons


leave your comment