Food Science Corner: Fat Crystallization
Last Updated on February 17, 2023
Quart, marble, opal, these are all kinds of crystals found across the Earth. They are beautiful with their unique geometries, colours and dazzling ability to reflect light in different ways. However, did you know that crystals can be edible too?
No, I don’t mean the stones.
Instead, I am talking about foods that you are familiar with in your everyday life. Fats, sugars and even proteins can all crystalize. An easy material to think about that can crystallize is water. Water when cooled under it’s melting point of 0°C transforms from a liquid to a crystalline solid. This crystalline structure is because of the shape of the water molecule and it’s ability to hydrogen bond.
Ice-cream’s texture benefits from water’s ability to crystallize at low temperatures. Ice-cream, as the name suggests contains ice which comes from the water found in milk. When producing ice cream you want it to have the smallest possible ice crystals (10 to 20 µm) as it has been shown that this leads to the best texture. Freezer burnt ice-cream has ice crystals greater than 50µm are known to cause a grainy texture. [1]
So if crystals exist in so many forms how does this actually happen? Well crystals form through the process of crystallization. Crystallization is the overall process of creating highly organized solid structures out of liquids [2] Understanding how crystallization works is essential to creating high quality products with predictable properties and shelf life.
Such as in the case chocolate. Have you ever looked at a chocolate and found that it has a weird white layer on top? Well that isn’t some microbial growth but instead it because the cocoa butter contained within has transformed into a crystal type which is undesirable.
In this article, I want to break down the basic science of crystallization from the context of fats (because that is my specialty!) However, this general process applies to other types of foods such as sugars.
Crystallization of Fats
Fat are materials that consist of a mixture of lipid components, consisting of 95% or more triacylglycerols. When a fat is supercooled past it’s melting point the highest-melting triacylglycerol begin to solidify resulting in the supersaturated state. This begins the process of crystallization. [3]
Related: What are Fats and Oils?
The process of crystallization can be divided into a different number of steps depending on where you are reading. Although arguably you could divide them up into two overall steps but to make this review simpler I will break them down into four.
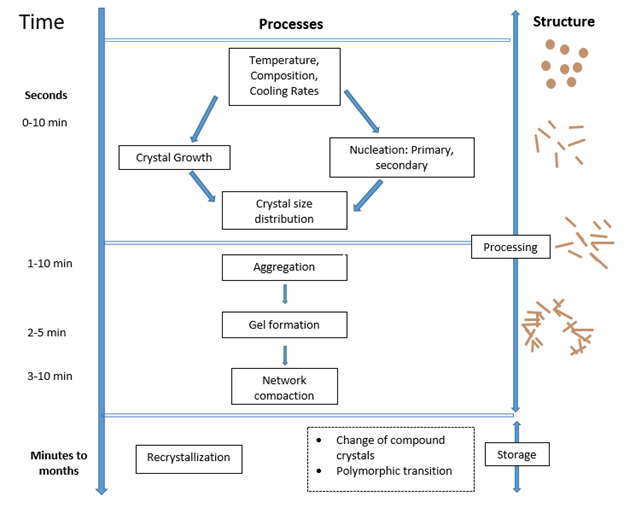
1. Supercooling
If you place a bottle of oil in a room, you wouldn’t expect it to spontaneously turn into a solid. This is because for crystallization to occur you need an outside force to push the process solidification process forward.
In the case of fats placing your oil in an environment which is colder than the highest melting triacylglyceride (TAGs) is typically sufficient enough to begin the crystallization process. I say typically because there are instances that this can happen. However, I want to keep it simple for this blog post. For a liquid-solid transition to occur you need to overcome an energy barrier which prevents a liquid from spontaneously turning into a solid. In the case of TAGs a sufficient driving force is cooling the oil under it’s melting point. Once this is achieved you can move onto the next step which is nucleation. [4]
2. Nucleation
During the nucleation step the TAG molecules start gather (aggregate) together into clusters. They are not quite yet a nucleus but instead solid embryos. These clusters will eventually become a stable nuclei but they need to reach a critical size in order to do that. A stable crystal will form only when the energy gained due to the heat of crystallization exceeds that required to overcome the surface energy required to increase the surface.
Factors which dictate whether a nuclei will start to get larger are temperature and the degree of saturation of the mixture. In essence if you have a lot of solid embryos in the mixtures it is more likely for nucleation to occur. According to classical theory there are three different types of nucleation: homogenous, heterogeneous and secondary nucleation. [2]
Homogeneous nucleation is a primary form of nucleation and is said to occur spontaneously in the bulk of the liquid, but does not occur in fats in practice. This is a theoretical type of nucleation and instead fats experience heterogenous nucleation. This nucleation is initiated by solid particles such as dust, container wall or seed crystals. Once primary nucleation has occurred the system can begin to nucleate via a second mechanism which is secondary nucleation which occurs when small pieces break from existing crystals and act as nuclei for further crystallization. [5]
3. Growth
Once the nucleus has formed, it can begin to grow. Have you ever seen an experiment with rock candy? How the crystals get bigger and bigger? This is what is happening here.
How fast the crystals grow is dependent on the amount of supercooling experienced by the system. Generally, a slower cooling rate results in slower growth compared to a higher cooling rate. Crystals do have the ability to grow larger through aggregation where the main mechanism for growth is the deposition of molecules and growth units onto the existing crystal lattice. During the phase change, there is a release of latent hear which must be transferred from the crystal surface. [4]
There is a lot of research that goes on in the fat crystallization space and it is a fascinating field of science. One of the reasons that I am pursuing a thesis on this topic!
4. Recrystallization
The final stage in the crystallization process is recrystallization which is defined as the change in number, size, shape, orientation, or perfection of crystals following completion of initial solidification.
Materials always want to be in the lowest possible energy state. Depending on how you crystallize something the first solid state you see is not always going to be the lowest energy state. A system getting to the lowest energy state doesn’t happen overnight- literally so the system well eventually reconfigure into this state.
Recrystallization occurs because small crystals are slightly more soluble or have a slightly lower melting point than larger crystals. These differences lead to the disappearance of the small crystals which than can grow on larger ones. All of this is due to the thermodynamics of the system.

Author: Veronica Hislop is a Master’s thesis student in the Molecular Science program at Ryerson University. She is also a career partner with FoodGrads and has work experience in the food processing industry working both in R&D and QA.
Currently, she is performing research on water-in-oil emulsions stabilized by fat crystals. When she is not following her scientific endeavors you can find her enjoying Japanese anime, manga and video games.
References
[1] Ice crystals in ice cream by Reuben Mattus
[2] Hartel, R. W. (2013). Advances in food crystallization. Annual Review of Food Science and Technology, 4(1), 277-292. https://doi.org/10.1146/annurev-food-030212-182530
[3] Co, E. D., & Marangoni, A. G. (2020). The phase space of crystallization: Modeling fat crystallization using thermodynamic and mass-transfer variables. Crystal Growth & Design, 20(3), 1628-1637. https://doi.org/10.1021/acs.cgd.9b01363
[4] Himawan, C., V. M. Starov, and A. G.F. Stapley. 2006. “Thermodynamic and Kinetic Aspects of Fat Crystallization.” Advances in Colloid and Interface Science 122(1–3): 3–33.
[5] Fat Crystallisation: mechanism and methods for studying- Powerpoint by Ralph E Timms


leave your comment