The Maillard Reaction: Unveiling Chemistry’s Tastiest Reaction
Last Updated on July 28, 2024
Envision a world where your cup of coffee tastes like grass, made from raw, unroasted coffee beans. Imagine having a piece of toast that not only lacks a nice golden-brown color but is starchy and almost flavorless. Thanks to the Maillard reaction, we can avoid experiencing this unfortunate reality!
Keep reading to learn more about the delicious chemical reaction that plays a vital role in transforming foods into more enjoyable and palatable products.
It’s about time to give this remarkable reaction some appreciation!
What is the Maillard reaction?
The Maillard reaction is also known as the “browning reaction.” This name comes from the appearance of the appetizing brown color caused by the application of sufficient heat to foods. The Maillard reaction is a result of the chemical reaction that occurs between amino acids and simple sugars. The reaction is named after the French scientist Louis-Camille Maillard, who first described it in 1912 while studying the science behind browning. +
The temperature requirement range is typically between 140°C to165°C (284°F – 329°F). As to why freshly baked bread smells different from roasted marshmallows depends on the structure of the molecules released as end products.
To have a better understanding of the Maillard reaction, take a look at this short video below.
How does the Maillard reaction make food tastier?
The flavour of roasted potatoes is undeniably more delightful than that of boiled ones, and similarly, roasted coffee beans offer a much more enjoyable flavour in comparison to when they’re raw.
Is there a particular reason for this difference in flavour?
The answer lies in the fact that different flavors and aromas are released during The Maillard Reaction depending on foods browned. Every food has unique combinations of chemical compounds contained. Therefore, allowing for a plethora of potential outcomes due to the Maillard reaction.
When foods are heated the amine groups from amino acids and carbonyl groups from simple sugars (monosaccharides) react together, forming hundreds of new compounds known as melanoidins which are molecules that contain high nitrogen levels and are brown in color.
The variation of these compounds depends end on the type of sugars and amino acids found in the food. A lot of these compounds are responsible for the delicious brown colour, flavour, and aromas released after heating. There are loads of different types of chemical compounds that we still need to identify, and that is due to the vast number of different possible combinations of sugars and amino acids.
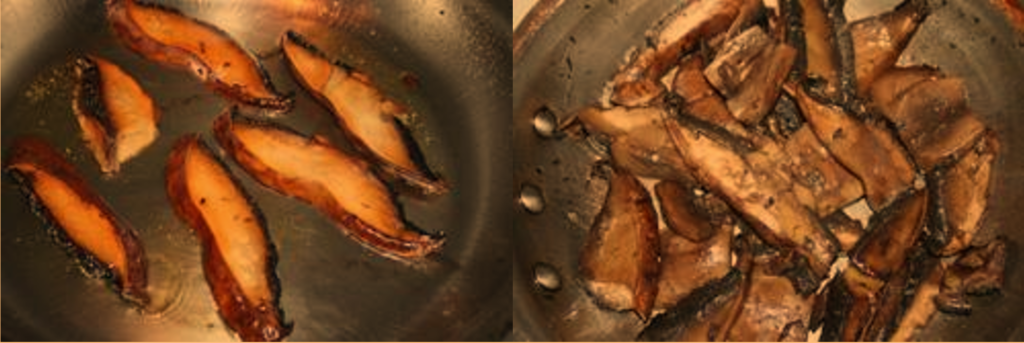
Flavor-producing aromatic molecules are produced by the Maillard reaction.
Through the contribution of Louis Maillard, the food industry and culinary world have benefited greatly from the Maillard reaction’s discovery! Though we can’t forget to thank those too which have figured out to create multiple tasty dishes without knowing the science behind them. With the perfect balance of these compounds, we’re able to produce mouth-watering flavors that can remain in our memory for ages.
Did you know? The Maillard reaction is not limited to just cooked food. It also plays a role in the producing of chocolate, beer and many other food and beverage products!
H2O: The enemy of the Maillard reaction
Why do cooking techniques such as braising and steaming not undergo the Maillard reaction?
This is because water significantly hinders the Maillard reaction due to its remarkable ability to absorb large quantities of heat. This lowers the overall temperature of the food undergoing cooking causing the Maillard reaction to occur inefficiently. From a molecular standpoint, the water molecules interfere with the interaction between the sugars and amino acids, thus lowering browning or completely preventing browning from occurring.
So how can you ensure the production the perfect brown color on whatever you cook?
The answer is to minimize the moisture content of the food as much as possible (enough to be palatable of course!). Drying can be done in many ways, such as patting the food dry, sun-drying, or even using an oven at a low temperature before cooking. It is also important to note that overcrowding when cooking allows food to release higher amounts of water; this delays browning and leaves less room for water evaporation.
The downside of browning
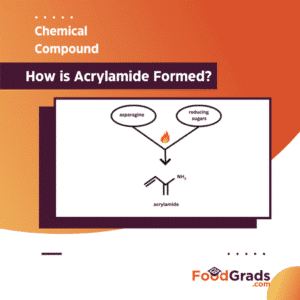
As there are no roses without thorns, we know that generally with heating, nutritional value decreases. Unfortunately, browning of foods in the Maillard reaction increases the release of a chemical known as Acrylamide. Acrylamide is a substance released by food products with high levels of carbohydrates and low in protein content when they undergo the Maillard reaction.
Acrylamide formation is primarily attributed to the interaction between simple sugars (eg. glucose, fructose) and the amino acid asparagine in most cases. Several research studies have established a link between Acrylamide and cancer in animals. Although there is no sufficient evidence regarding the risk posed by Acrylamide in humans, major organizations such as the FDA and WHO recommend reducing the consumption of Acrylamide-forming foods after undergoing the Maillard reaction. High levels of Acrylamide are typically released by starchy foods such as potatoes, bread, and biscuits when undergoing heated at specific temperature range.How is Acrylamide Formed?
The future
Chemists and researchers are currently working on identifying and exploring the possible aromatic compounds released by the Maillard reaction.
As our knowledge of food chemistry expands and technology advances, we can anticipate an increase understanding of the Maillard reaction. This could possibly allow us to acquire the ability to enhance flavor control through tools such as artificial intelligence, or perhaps even discover methods to mitigate the health risks associated with this reaction!



leave your comment